AUSTIN, Texas — On Monday, Total Men's Primary Care started allowing patients to get tested for COVID-19 antibodies. Patients who are no longer symptomatic can make an appointment on the company's website to get an antibody test.
“These tests are not FDA-approved yet, the manufacturers are working through that process," Robert Sek, the founder of Total Men's, said.
The FDA approved only one test for antibody testing -- Cellex. On April 1, the federal agency approved Cellex only for emergency use during the COVID-19 pandemic.
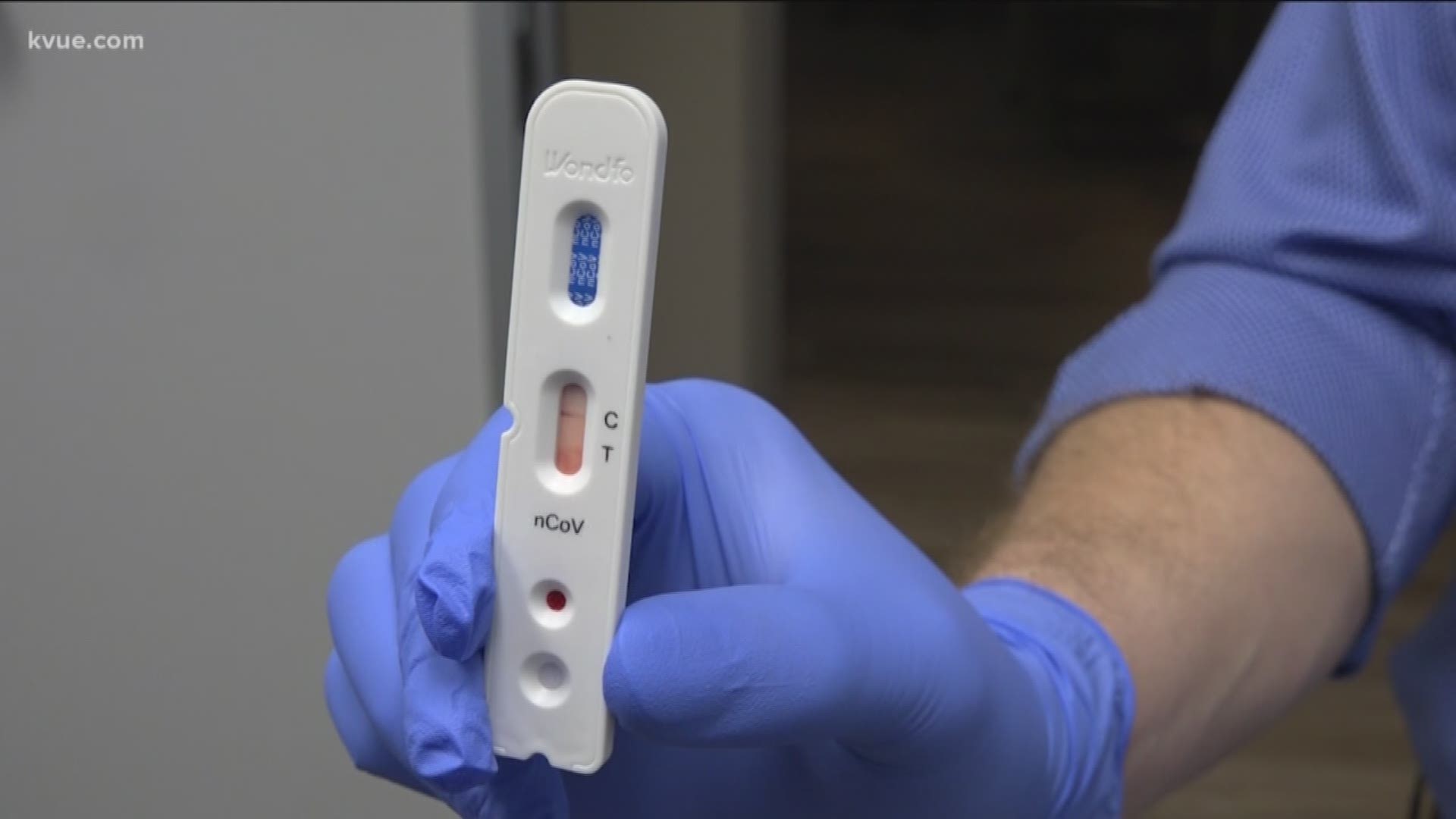
However, Sek's company is using a test called Wondfo SARS-CoV-2 Antibody Test. Wondfo, according to the Center for Health Security at Johns Hopkins University, has been approved for diagnostic use to test for antibodies in China. The approval came down from China's equivalent of the FDA: the National Medical Products Association. It's one of 10 antibody tests available for testing in some countries outside of the U.S., according to CHS.
“We probably looked at 30 different [tests]," Sek said. "We tried reaching out to [Cellex] on a daily basis. The reality is you can’t get them at all right now.”
RELATED:
Because Wondfo has not been validated by the FDA, Sek's company needs to make clear a handful of stipulations when somebody wants to get tested for COVID-19 (also called SARS-CoV-2) antibodies:
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43 or 229E.
If the above bullet points are met by healthcare providers and test manufacturers, the FDA has said it will not object to the use and development of other antibody tests.
Late Sunday night, Total Men's posted on Facebook that the company would be open for COVID-19 antibody testing appointments. The clinic has 24 locations -- 14 in and around Austin and 10 in the Dallas-Fort Worth area.
Antibodies from people who have recovered from the virus can help those who are still fighting the virus.
Sek said results from Wondfo show up in about 15 minutes. Notably, these tests are not to determine if a person has coronavirus, but if they previously had it and developed antibodies. It does not determine if somebody is still contagious or not.
The company has a limited supply of tests. Sek said they can perform a few hundred per day, but is unsure how long the tests would last.
According to Sek, the tests are not free: $49 with major insurance, $199 without. Total Men's, while normally designated for men, is available to women and children looking to get tested for antibodies as well.
PEOPLE ARE ALSO READING: